A Comparative Study of Toxic Thiocynate Ion in the Saliva of Smokers and Non-Smokers
Lynn Krebs
ABSTRACT
For years there have been warnings against smoking and its detriment to
one�s health. I pose the
question, is it just as dangerous to be the one who is exposed to the secondhand
smoke as it is to be the tobacco user? To
answer this question, the relative amounts of toxic thiocyanate ion in saliva
obtained from a variety of individuals are compared.
In order to determine these amounts, absorption spectrophotometry is used
in the analysis of the FeSCN2+ complex.
This question is of importance because it offers support for the argument
that smoking is hazardous to everyone�s health.
INTRODUCTION
This study intends to analyze by spectrophotometric methods, the amount
of thiocyanate ion present in eleven subjects with differing exposures to
tobacco smoke. It was reported that
high concentrations of HCN, a toxic gas, are present in cigarette smoke (Surgeon
General, 1990, 35). The ingestion
of this smoke leads to the formation of the thiocyanate ion
(SCN-) in the liver. Since
the HCN is present in the cigarette smoke and not directly in the cigarette,
this can be a potential hazard to the nonsmoker as well, in the form of
secondhand smoke. Secondhand smoke is �a dilute mixture of mainstream smoke
exhaled by smokers and sidestream smoke from the burning end of a cigarette or
other tobacco product and it is chemically similar to the smoke inhaled by
smokers� (www.tobacco.org 4/11/01).
This secondhand smoke accounts for approximately 3,000 lung cancer deaths
annually and 37,000 heart disease death in non-smokers (American Lung
Association Fact).
Tobacco smoke contains HCN gas. Once
this gas is ingested it is dissociated into its components according to the
following equilibrium equation:
HCN(aq)
��
H+(aq) + CN-(aq)
Equation 1
The
CN- ion is then
converted into the thiocyanate ion when it reaches the liver.
The thiocyanate ion reacts with the Fe3+ ion to yield the
FeSCN2+ complex that can be detected spectrophotometrically.
In order to detect the amount of thiocyanate present in the saliva, the
clear solution collected from centrifuging the saliva and a 0.0038 M solution of
Fe(NO3)3 in 1M aqueous HNO3 are mixed together.
When combined, the iron forms a complex with the thiocyanate ion as
stated below.
Fe3+(aq) + SCN-(aq) �
FeSCN2+(aq)
Equation 2
This
complex exhibits a red-orange color. The
deeper the color, the more concentrated the solution is.
This color difference allows for the analysis by visible spectroscopy.
The UV-VIS Spectrophotometer is used to measure the absorbances of
colored solutions at a variety of wavelengths.
The darker the color is, the higher the absorbance value is.
In this study, the absorbances of several solutions consisting of varying
concentrations of FeSCN2+ complexes are measured at 447 nm to
construct a standard curve. The
unknown concentrations of FeSCN2+ ion in saliva samples are
determined using the linear relationship between absorption and concentration as
established by the standard curve.
The results of this study show that the greater the exposure to the smoke
is, the higher the concentration of thiocyanate ion is in saliva.
Since smokers are exposed to more tobacco smoke than nonsmokers, it seems
that they should have an initial SCN- concentration that is higher
than nonsmokers and therefore a higher absorbance reading.
The saliva samples are collected from subjects with varying smoke
exposure, including smokers, nonsmokers, nonsmokers who live and/or work with
smokers, and a tobacco chewer. The
results are fairly consistent with other laboratory data, which shows that there
is a linear relationship between exposure to tobacco smoke and concentration of
the thiocyanate ion in the body.
MATERIALS
Spectronic Instruments Genesys 5 Spectrophotometer
Quartz cuvettes
0.0038 M Fe(NO3)3 � 9 H20 in 1 M
aqueous HNO3
0.0002 M KSCN
Tabletop centrifuge
Other basic laboratory equipment
METHODS
In conducting this lab, a standard curve is obtained using solutions
listed in Table 1. Keeping the
amount of Fe3+ ions constant, as well as the total volume, and
increasing only the amount of SCN- ions added, an increasing amount
of FeSCN2+ complex is formed in standard solutions.
|
|
Volume
(ml) |
Volume
(ml) |
Volume
(ml) |
Concentration
(M) |
|
Test
tube no. |
0.0038
M Fe(NO3)3 |
0.0002
M KSCN |
H2O |
FeSCN2+ |
|
0 |
5 |
0 |
5 |
0 |
|
1 |
5 |
1 |
4 |
1.33
x 10-5 |
|
2 |
5 |
2 |
3 |
2.65
x 10-5 |
|
3 |
5 |
3 |
2 |
3.99
x 10-5 |
|
4 |
5 |
4 |
1 |
5.27
x 10-5 |
|
5 |
5 |
5 |
0 |
6.58
x 10-5 |
Table I - Standard Curve Compositions
Once
the standard curve is obtained, the saliva samples are tested.
In order to prepare the samples for the experiment, they should be
centrifuged at 3000 rpm for at least ten minutes. The final solutions are obtained by mixing 0.5 ml of the
resulting clear solution with 0.0019 M Fe(NO3)3 to give a
total volume of 10 ml. If
thiocyanate ion is present, the clear solution becomes orange tinted. The more thiocyanate ion that is present in the sample, the
darker the orange color. After all
samples are prepared, the absorbances are measured at 447 nm against 0.0019 M
Fe(NO3)3, which is used as a blank.
The results are presented in the following section.
RESULTS
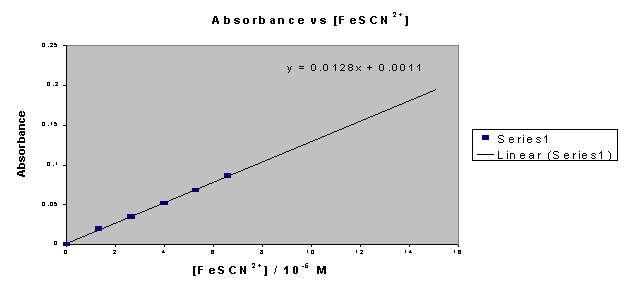
The absorbances of the standard solutions measured at 447 nm are
presented in Table II.
|
Concentration
FeSCN2+ (M) |
Absorbance |
|
0 |
0 |
|
1.33
x 10-5 |
0.020 |
|
2.65
x 10-5 |
0.035 |
|
3.99
x 10-5 |
0.052 |
|
5.27
x 10-5 |
0.068 |
|
6.58
x 10-5 |
0.086 |
Table II - Standard Curve Absorbances
The
following graph is the standard curve, which was used to calculate the FeSCN2+
complex concentration of each saliva sample according to its absorbance reading.
That information was further used to calculate the SCN-
concentration in the original saliva samples.

Graph 1 - Standard Curve
The data for the saliva samples
are presented in Table III.
|
Sample ID Number |
Absorbance |
FeSCN2+ |
|
1 |
0.050 |
3.82
x 10-5 |
|
2 |
0.053 |
4.08
x 10-5 |
|
3 |
0.055 |
4.21
x 10-5 |
|
4 |
0.073 |
5.62
x 10-5 |
|
5 |
0.078 |
6.01
x 10-5 |
|
6 |
0.098 |
7.57
x 10-5 |
|
7 |
0.179 |
13.9
x 10-5 |
|
8 |
0.041 |
3.12
x 10-5 |
|
9 |
0.090 |
6.95
x 10-5 |
|
10 |
0.091 |
7.05
x 10-5 |
|
11 |
0.159 |
12.3
x 10-5 |
Table III - Saliva Sample Data
The FeSCN2+ concentration for each saliva sample is calculated
from the absorbance values measured using the equation of a line for the
standard curve (y = 0.0128x + 0.0011). The
initial SCN- concentrations in saliva were then calculated using the
formation constant for the FeSCN2+ complex formed in equation 2 as
follows:
Kf
= [FeSCN2+] / [Fe3+ - FeSCN2+][SCN-
- FeSCN2+]
Equation 3
The
SCN- concentration was then multiplied with the initial dilution
factor to get the SCN- concentration in the saliva.
The results are presented in the following table.
|
Sample
ID Number |
Smoke
Exposure |
SCN-
in Saliva (M) |
|
1 |
Nonsmoker
(lives with nonsmokers) |
1.16
x 10-3 |
|
2 |
Nonsmoker
(lives with nonsmoker) |
1.24
x 10-3 |
|
3 |
Nonsmoker
(lives with nonsmoker) |
1.27
x 10-3 |
|
4 |
Nonsmoker
(works with smokers) |
1.71
x 10-3 |
|
5 |
Nonsmoker
(lives with smoker) |
1.83
x 10-3 |
|
6 |
Nonsmoker
(lives with smoker) |
2.30
x 10-3 |
|
7 |
Nonsmoker
(lives with two smokers) |
4.34
x 10-3 |
|
8 |
Tobacco
Chewer (lives with nonsmoker) |
9.42
x 10-4 |
|
9 |
Smoker
(� pack a day) |
2.12
x 10-3 |
|
10 |
Smoker
(� pack a day) |
2.14
x 10-3 |
|
11 |
Smoker
(1 � pack a day) |
3.78
x 10-3 |
Table IV - Saliva Sample Results
As
is illustrated by this data, smokers and nonsmokers who live with smokers have
higher absorbances at 447 nm, i.e., a higher concentration of thiocyanate ion,
than nonsmokers with little exposure to tobacco smoke.
This data indicates that nonsmokers who are exposed to second hand smoke
are in equal or more danger of ingesting the toxic HCN gas present in cigarette
smoke.
Graph
II contains both the standard curve as well as all saliva samples.
This further illustrates the linear relationship between the amount of
FeSCN2+ complex present and the absorbance reading.
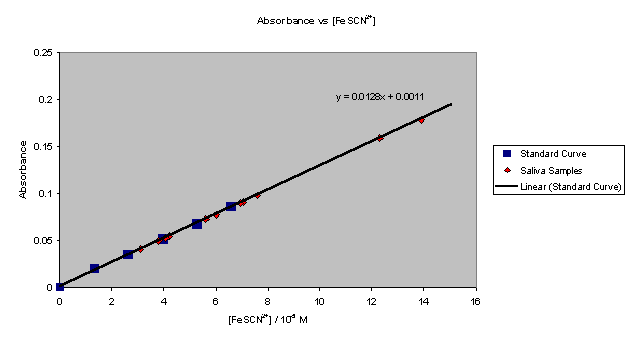
Graph II � Standard Curve with Saliva Samples
The
graph seems to indicate that the last two samples have a complex amount that is
much greater than the other samples, while in reality they are approximately
twice the amount as the rest of the sample group.
DISCUSSION
This study shows that it is possible to determine the amount of HCN present in tobacco smoke using a simple spectrophotometric technique. In this experiment, the absorbances of saliva samples treated with Fe3+ are used to qualitatively and quantitatively compare relative amounts of thiocyanate ion in smokers, nonsmokers, and nonsmokers who are exposed to second hand smoke.
Table IV illustrates the relationship between tobacco smoke exposure and thiocyante ion concentration. The highest concentrations of thiocyanate ion belong to the donor who smokes the most and the donor who lives with two smokers. They have the most exposure to the tobacco smoke and therefore, because of the linear relationship between the two variables, they have the highest thiocyanate ion concentrations. As the sample's exposure to smoke increases, the thiocyanate ion concentration also increases. In fact, with the exception of the nonsmoker who lives with two smokers, nonsmokers have lower thiocyanate concentrations than smokers. The lowest thiocyanate ion concentration was surprisingly from the tobacco chewer. It would seem that since the tobacco chewer has direct contact with the tobacco, that their thiocyanate ion concentration would be fairly high. This is not the case though. The thiocyanate ion is formed from the conversion of the HCN gas in the smoke produced from the tobacco not from the tobacco itself. It can also be concluded from the direct relationship between smoke exposure and thiocyanate ion concentration that the tobacco chewer has the lowest amount of exposure to secondhand smoke.
It is clear from the results of the saliva samples that there is a dangerous relationship between the relative amount of tobacco smoke and the amount of thiocyanate ion present in their body. While most of the subjects in this study who are nonsmokers did not have a higher amount of thiocyanate ion as compared to the tobacco smoker, it is clear that the exposure to secondhand smoke can be just as harmful as the direct inhalation of it. It is reported that if a nonsmoker is around a person who smokes a pack of cigarettes during the day, the nonsmoker�s exposure is comparable to his or her smoking half of that pack of cigarettes. (www.americanheart.org 4/11/01). The results of this study suggests that the secondhand smoker�s exposure is even higher than previously thought and can be comparable to the total amount of exposure a smoker experiences.
In the interpretation of the results, one should also consider factors such as other possible sources of cyanide. It is known that certain foods, particularly leafy vegetables, some nuts, and certain drinks like beer contain cyanide. (Surgeon General, 1986, 202). A more careful study requires monitoring the diet of each subject so that the possible competing sources of thiocyanate ion are known. We intend to confirm the results of this study by taking into account the other possible sources of cyanide present in the subjects� diets in a follow-up experiment. Another complication that must be considered is saliva activity. It is suggested that the saliva flow rate at the time of sampling can influence the thiocyanate concentration present in the sample (www.ucn.es 2/21/01).
HCN along with a number of other carcinogenic compounds can lead to many health problems. As was mentioned earlier, the hazards of smoking include lung and heart disease. They can also lead to mouth cancer, dental problems, asthma, and emphysema, as well as several other medical problems. While the effects are greater in smokers, it is evident from the results of this study as well as many others, that secondhand smoke is detrimental to one�s health.
Works Cited
1. C. Matheus and K.E. Van Holde; Biochemistry, The Benjamin/Cummings Company, 1990.
2. Lahti, Markku, Vilpo, Juhani, and Hovinen, Jari, Spectrophotometric Determination of Thiocyanate in Human Saliva. Journal of Chemical Education, Vol. 76, No. 9, September 1999. pgs 1281-1282.
3. Surgeon General, The Health Consequences of Involuntary Smoking: a report of the Surgeon General. 1986. pg 202-203.
4. Surgeon General, The Health Benefits of Smoking Cessation: a report of the Surgeon General. 1990. pg. 35.
4. Media Advisory on Secondhand Study Published in Journal of American Medical Association. http://www.americanheart.org/Whats_News/AHA_Science_Advisories/2HandSmoke.html
5.
American Lung Association Fact Sheet: Secondhand Smoke and Children.
http://www.lungusa.org/tobacco/secondkids_factsheet.html
6.
Setting the Record Straight: Secondhand Smoke is A Preventable Health
Risk. http://www.tobacco.org/Documents/9406EPA.html
7.
Clinical and Community Psychology. http://www.ucn.es/info/Psyap/231CAP/abstract/clinical4.html
Acknowledgment:
Special thanks to Dr. Feza Ozturk, Amie Touchette, Catharine Graham, Mike Melvin, Lindsay Braun, Gina Heinen, Aaron Krebs, Lyndon Mumm, Heather Heenan, and Lynn Hollenkamp for their contributions to this experiment.