The effects of varying carbohydrate levels on cognitive learning in rats
Sara Rubenacker
ABSTRACT
What we eat has a bigger impact on us than just satiating hunger and providing us with energy. Dietary components have the ability to affect our moods and brain functioning. This study focuses on the role of carbohydrates on memory in rats. Adult Sprague-Dawley rats were divided into two groups; the control group was fed a regular diet of laboratory chow (60% carbohydrate, 20% protein, 20% fat), while the experimental group was fed a low carbohydrate chow (30% carbohydrate, 50% protein, 20% fat). Cognitive learning was assessed by using a Morris-type water maze task, consisting of three training days and one test day. Comparisons were made for time taken to reach platform (latency), number of crossings, and time spent in target quadrant. Learning occurred in both groups during the training period. While no difference between groups was found for the tasks of latency or number of crossings, statistical analysis for time spent in target quadrant found that a difference existed between the groups for this task. These findings indicate the possible role of carbohydrate consumption on memory modulation.
Introduction
The brain is a highly active metabolic organ, using 20% of the body�s energy consumption (Benton 2003). The energy is utilized in the biosynthesis of several regulatory compounds and the transport of molecules to maintain proper brain functioning. The brain is unable to store significant amounts of energy to support these functions and must rely on circulating fuel supplies, mainly in the form of glucose. Glucose maintains metabolism in the brain and is used in the synthesis of amino acids, peptides, lipids, nucleic acids, and neurotransmitters. Therefore, dietary components that provide glucose may help the brain function at a more optimal level.
Due to the role of glucose in memory formation, dietary carbohydrates may play a crucial role in cognitive functioning. The ingestion of dietary carbohydrates rapidly increases levels of blood glucose (Benton et al. 2003), which may enter the brain through the blood brain barrier. Increased provision of glucose may be associated with better memory modulation. Memory may also be affected by the type of carbohydrate, whether simple or complex, and the ratio of carbohydrate to protein or fat in the meal (Fischer 2002). Memory may also be improved to a greater extent by foods that furnish slowly available glucose rather than rapidly available glucose.
Glucose�s significance has been studied in several different experiments, (Salinas 2004, Benton et al. 2003, Gold 1995) and many have concluded that memory enhancement may be tied to the glucose metabolism pathway. Acetyl CoA, a metabolite of glucose, is a precursor in the synthesis of acetylcholine, which plays a role in memory (Benton 2003). Alternatively, glucose may play a role in the endogenous system of the brain that regulates the process of memory formation (Salinas 2004). Studies conducted by Gold (1995) have shown epinephrine�s role in the enhancement of memory. Learning a new task and forming memories are taxing events, and the body utilizes epinephrine to combat stressful situations (Korol 1998). Epinephrine, in turn, causes an increase in blood glucose levels which may explain the reason for memory enhancement.
With the past popularity of low-carb diets, one has to wonder if those who followed low-carb regimens were sacrificing their cognitive health in order to drop a few pounds. To better understand the link between dietary carbohydrates and cognitive functioning I investigated the effects of varying carbohydrate levels on memory modulation in rats.
Materials and Methods
Adult Sprague-Dawley rats purchased from Harlan Biological Supply (Indianapolis IN, USA) were divided into two groups consisting of three female and three male rats. The rats were housed in four plastic cages with three rats of the same gender to a cage in a laboratory setting. The control group was fed a regular diet of laboratory chow (60% carbohydrate, 20% protein, 20% fat), while the experimental group was fed a low carbohydrate chow (30% carbohydrate, 50% protein, 20% fat). Both diets were obtained from Dyets, Inc., Bethlehem, PA. Rats had access to food and water ad libitum. Diets were maintained for a period of ten weeks.
Spatial learning was assessed by conducting a Morris-type water maze task (Morris 1984) using an 8 ft diameter circular pool divided into four equal quadrants placed in a room with consistent spatial cues (a bulletin board, cabinets, and door). The pool was filled with water to a depth of 11 cm. An escape platform (10 cm diameter) was placed 1 cm below the water surface in the center of one of the quadrants. The platform provided the only escape from the water and was in the same location throughout the training period. Four different starting positions were established before the trials began. On each of the training days, all four starting positions were used once for each rat, (i.e., four training sessions per day), with an inter-trial interval period of 15 minutes.
For each trial, the rat was placed in the water at one of the starting points. If the animal failed to find the platform within 60 s it was gently conducted to the platform by the experimenter and allowed to stay on the platform for 15 s. Animals were trained for three days. Twenty-four hours after the last training session, I again placed the rats in the water maze for 1 min. to test their ability to remember the location of the escape platform. Before the test session, the escape platform was removed. For each rat, I recorded the number of seconds it took them to first cross the original location of platform (latency), the number of times they crossed the original location of the platform over the course of the test, and the time they spent in the target quadrant.
Results
Learning over the length of the training period
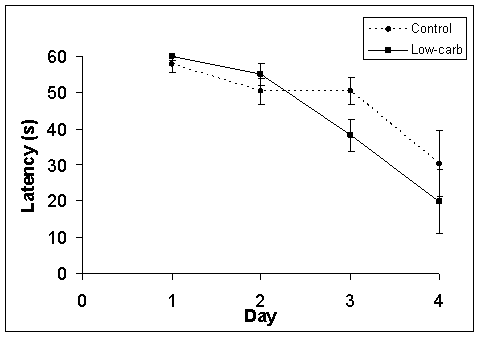
Both groups showed signs of learning during the training period (Fig. 1). Latency to finding the platform decreased for both groups significantly but in different ways. While both groups improved, the low-carb group only saw a significant decrease in their latency between day 2 and day 3 while the control group saw a more immediate improvement from day 1 to day 2.
Affect of diet on learning
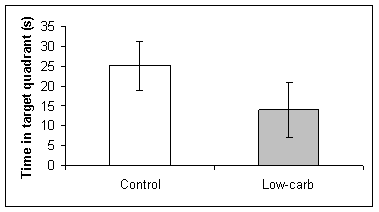
The amount of time the two groups spent in the target quadrant during the test session were significantly different (t-test, t=2.713, df=8, P=0.027, Fig 2A). Based on the results of the t-test, the control group spent more time in the target quadrant than the low-carbohydrate group.
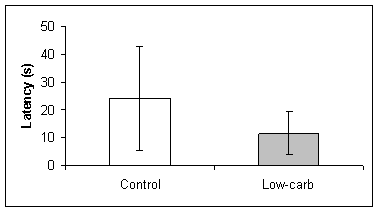
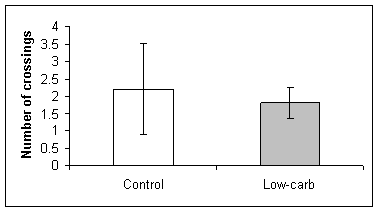
No effects of dietary carbohydrates were found for the latency or number of crossings during the retention test. The control group and low-carbohydrate group presented the same latency in reaching the original location of the platform (t-test, t=1.391, df=8, P=0.202, Fig 2B). The control rats and low-carbohydrate rats also crossed the original location of the platform the same number of times (t-test, t=0.649, df=8, P=0.535, Fig 2C).
Discussion
Previous studies indicate that low-carbohydrate consumption has the ability to alter many aspects of brain functioning due to depleted levels of circulating glucose. My study focused on the effects of carbohydrates on spatial learning in rats. By comparing latency levels, number of crossings, and time spent in target quadrant, I was able to compare levels of learning between the two groups.
One concern when conducting this kind of study is whether any of the rats are learning. Latency times decreased each day of the training period for both groups, indicating that the rats were learning the position of the platform. Latency time did not level off, however, which suggests that a longer training period may have been needed to decrease latency time even further.
Control rats spent more time in the target quadrant than rats following a low-carbohydrate regimen, indicating that the amount of dietary carbohydrates did influence ability to perform in the water maze task. Control rats may have had more glucose available for brain metabolism, resulting in quicker memory modulation. Stores of glucose in the low-carbohydrate group may have been depleted to a critical level which hindered the rate of cognitive functioning, resulting in the discrepancy between groups. These results are agreement with other studies on the roles of glucose and carbohydrates on cognitive functioning (Gold 1995, Lieberman 2002).
No significant differences were found between the groups for latency in reaching the platform position and number of crossings. These results may be explained by the training period being too brief. A longer training period was necessary to produce more reliable results. Alternatively, the low-carbohydrate group may have been using alternate sources of fuel for brain metabolism, which may have put them at par with the control group in terms of brain metabolism which resulted in similar outcomes.
These results reinforce carbohydrate�s role in memory modulation through glucose availability. While both groups showed evidence of learning, the group with more dietary carbohydrates spent more time in the target quadrant than the low-carbohydrate group suggesting that the control group knew the vicinity of the platform�s original position to a greater extent than the low-carbohydrate group. This result enforces carbohydrate�s role in the task of spatial learning.
Acknowledgements
I would like to thank Dr. Michael Henshaw, Dr. David Jennings, and Dr. Robb Van Putte for their suggestions, wisdom, and patience throughout the production of this paper. I am also grateful to my classmates in Sr. Thesis who helped me see the humor at all times. Finally, I would like to thank Shawn Stratman for his help with my project and the McKendree inter-library loan staff for their tireless efforts to secure literary sources needed for this paper.
Bibliography
Al-Mudallal AS, Levin BE, Lust WD, et al. 1995. Effects of unbalanced diets on cerebral glucose metabolism in the adult rat. Neurology 45(12): 2261-5.
Benton D, Nabb S. 2003. Carbohydrate, memory, and mood. Nutrition Reviews 61(5): 1-8.
Benton D, Owens DS, Parker PY. 1994. Blood glucose influences memory and attention in young adults. Neuropsychologia 32: 595-607.
Benton D, Ruffin M-P, Lassel T, et al. 2003. The delivery rate of dietary carbohydrates affects cognitive performance in both rats and humans. Psychopharmacology 166: 86-90.
Evans M, Amiel SA. 1998. Carbohydrates as a cerebral metabolic fuel. J Pediatr Endocrinol Metab 3(1): 99-102.
Falkner F, Tanner JM. 1979. Neurobiology and nutrition. New York: Plenum Press.
Fischer K, Colombani PC, Langhans W, et al. 2002. Carbohydrate to protein ratio in food and cognitive performance in the morning. Phy Bhvr 75(3): 411-24.
Geisler MW, Polich J. 1992. Food consumption and memory performance. Psychophysiology 29: 76-85.
Gold PE. 1995. Role of glucose in regulating the brain and cognition. An J Clin Nutr 61(4): 987-95.
Gold PE, Vogt J, Hall JL. 1986. Glucose effects on memory: behavioral and pharmacological characteristics. Behav Neural Biol 46(2): 145-55.
Hall JL, Gonder-Frederick LA, Chewning WW, et al. 1989. Glucose enhancement of performance on memory tests in young and aged humans. Neuropsychologia 27 (9): 1129-38.
Kaplan RJ, Greenwood CE, Winocur G, Walover TM. 2000. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. Am J Clin Nutr 72 (2000): 825-36.
Kaplan RJ, Greenwood CE, Winocur G, Walover TM. 2001. Dietary protein, carbohydrate and fat enhance memory performance in the healthy elderly. Am J Clin Nutr72: 825-836.
Korol DL, Gold PE. 1998. Glucose, memory, and aging. American Journal of Clinical Nutrition 67(4): 764S-71S.
Lieberman HR. 2003. Nutrition, brain function and cognitive performance. Appetite 40(3): 245-55.
McGaugh JL. 1983. Hormonal influences on memory. Ann Rev Psychol 34: 297-323.
Morris R. 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47-60.
�z G, Henry P, Seaquist ER, et al. 2003. Direct, noninvasive measurement of brain glycogen metabolism in humans. Neurochemistry International 43(4/5): 323-30.
Park CR. 2001. Cognitive effects of insulin in the central nervous system. Neurosci Biobehav Rev 25: 311-323.
Rodriguez WA, VanAusdle LR, Dhanens K, Mondragon AN. 1993. Glucose attenuates recently reactivated memories. Psychobiology 21: 93-100.
Salinas JA, Gold PE. 2004. Glucose regulation of memory for reward reduction in young and aged rats. Neurobiology of Aging 26(2005): 45-52.
Susser M. 1989. The challenge of causality: human nutrition, brain development, and mental performance. Bull N Y Acad Med 65(10): 1032-49.
Vicente E, Boer M, Leite M, et al. 2004. Cerebrospinal fluid S100B increases reversibility in neonates of methyl mercury-intoxicated pregnant rats. NeuroToxicology 25 (2004): 771-777.
Ward E. M. 2004. Boost your brain, sharpen your memory by eating smart, keeping healthy. Env Nutr 27(8): 1-3.
Figure 1. Change in latency over training period and test period. Days 1-3 refer to the training period. Day 4 is the test day. Both groups showed spatial learning over the course of the experiment period.

Fig. 2 Spatial learning in a water maze by rats on normal and low carbohydrate diets. Learning was evaluated by (A) the time spent in the target quadrant, (B) the latency (s) to finding the platform location, and (C) the number of times the rat crossed the location of the platform. No statistical differences were found in the latency or number of crossings for each group, but control rats spent significantly more time in the target quadrant (t-test, t=2.713, df=8, P<0.027).
(A)
(B)
(C)
©