Determination of the Stoichiometry of Complex Formation
Between Transition Metal Ions and Tyrosine Using UV Absorption Spectrophotometry
Samantha
Harriss
Abstract
L-tyrosine and various transition metals are
vital to biological functions. The coordination of L-tyrosine and Fe(II), Cu(II),
Cd(II), and Zn(II) was investigated by ultraviolet absorption spectrophotometry.
Using the mole ratio method, a 2:1 complex of Cu(II):tyrosine was reconfirmed
and a new 2:3 complex of Cu(II):tyrosine was proposed. Fe(II) ions seemed to
complex to tyrosine in the molar ratios of 2:1 and 2:3 Tyr:Fe(II). Cd(II) and
Zn(II) did not form complexes of spectroscopic sensitivity with tyrosine and
were used as controls. Proposed structures of the Fe(II) and Cu(II) complexes
are given.
Introduction
The amino acid L-tyrosine and various
transition metals are important in the biological functions of humans, animals,
and plants. L-tyrosine is one of the twenty major amino acids and is considered
an essential amino acid. This amino acid is synthesized in the human body from
phenylalanine and is a direct precursor of various hormones, biogenic amines,
and neurotransmitters. It is used by the thyroid and adrenal glands to
synthesize thyroid hormones and adrenaline, respectively [3]. Tyrosine
metabolizes to products such as melanin, the pigment found in hair and skin,
estrogen, and encephalin. Tyrosine, a precursor to dopamine, also plays a role
in the neurotransmission signaling and regulation of depression. Other
non-human organisms utilize tyrosine in their biological processes. Sea squirts
contain a transport protein, transferrin that contains two tyrosine residues in
the terminal lobe that aid in the coordination of vanadium [5]. Much of the
tyrosine used in the laboratory is prepared from plants, namely sugar beets.
Other plants such as sweet potatoes contain tyrosine in the active sites of
enzymes that catalyze the hydrolysis of phosphoric acid esters and phosphoric
acid anhydrides [4].
Inorganic elements like
transition metals are vital to the proper functioning of the body�s processes.
Metal ions have the ability to form strong bonds and be stable in more than one
oxidation state. Iron has a major role in biological reactions. Iron in the
blood chelates with protoporphyrin to form heme, a prosthetic group of proteins
such as myoglobin, hemoglobin, catalase, peroxidase, and cytochrome c. It also
plays a role in enzyme activity. Enzymes called oxygenases catalyze the
cleavage or degradation of aromatic amino acid rings in biological systems. For
example the degradation of phenylalanine begins with its hydroxylation to
tyrosine, a reaction catalyzed by phenylalanine hydroxylase. The active site of
this enzyme contains iron that is not part of heme or an iron-sulfur cluster
[1]. Zinc is found only in the 2+ state in biological systems. It is known to
form complexes with amino acids such as L-serine, L-aspartic acid, L-lysine, and
L-phenylalanine [8]. In a previous study, a zinc-tyrosine complex was
synthesized in the core of encapsulated dendrimers [9]. Copper ions form a
complex with tyrosine that inhibits demethlyation and stimulates oxidation of
microsomal pigments in hepatic cells [12]. The complex acts as an electron
acceptor which stimulates the oxidation process. Copper ions have also been
found to be required in the metabolic transformation of tyrosine [3]. Cadmium
is a naturally occurring transition metal found in the earth�s crust. Although
it is naturally occurring in the earth�s crust, it produces toxic effects in
humans. Cadmium accumulates in the kidneys and at high concentrations can cause
kidney failure. The primary route for cadmium intake is ingestion. Trace
amounts are found in food products due to the use of phosphate fertilizers used
on agricultural soil. Cigarette smoking is also a means of cadmium exposure.
Complex formation of cadmium with tyrosine could be a means of elimination of
this toxic metal.
Tyrosine has three important
components: a carboxylic group, an amino group, and a phenolic group. It is one
of three amino acids with a bulky, uncharged, aromatic side group which gives
tyrosine an absorption spectrum that can be observed by ultraviolet and visual
spectrophotometry. The remaining three groups give tyrosine the pKa
values of 2.2 (-COOH), 9.1 (-NH3+), and 10.5 (-OH). Due
to these pKa values, tyrosine has the ability to exist in the
monoprotonated form, HL, over a pH range of 2.7 to 8.5 where neither the
phenolic group nor the amino group has completely released their protons. The
carboxylic group is completely deprotonated over this pH range. In a study by
Renzo Carta, the solubility of tyrosine was examined at various pH values from
0.0 to 13.0 in aqueous solutions [2]. The experimental conditions of Carta�s
study were similar to other reports because a broad pH range [3, 10, 11] and an
aqueous solution [2, 3, 5, 10, 14-16] were used. Tyrosine concentrations in
solution ranged from as low as 10-6M [11, 15] to a high of 10-2M
[14].
Many studies have been done
showing that tyrosine has the ability to covalently bond to transition metals as
well as bond noncovalently to alkali metals [3, 4, 10, 11, 13-16]. The majority
of the coordination occurs at the amino nitrogen or carboxylic oxygen of
tyrosine [10, 13, 14, 16] but is also believed to occur at the phenolic oxygen
[13, 16]. The bulk of the tyrosine-metal studies have involved complexes with
transition metals (binding ratio M:L): Cu2+ (1:2) [10, 15], Y3+
(1:1 and 1:2) [14], Hg2+ [11], Zn2+ [9] and Fe3+
[4]. Tyrosine does not directly bond but is involved in the coordination of the
transition metal, vanadium [5]. Other studies have included metals from the
lanthanide series (binding ratio M:L): La3+ (1:1) [14], Ce3+
(1:1) [14], and Eu3+ (1:2) [16]. Victor Ryzhov et al. performed a
unique study of the ability of tyrosine to noncovalently bond with alkali metals
Na+ and K+ [13]. However no studies have reported
findings concerning the direct bonding of tyrosine with Fe2+, Cd2+,
which are two of the four ions used in this study.
Tyrosine-metal complexes have been studied
using a wide variety of techniques. Some analytical techniques used include
voltammetry [15], amperometry [11], and potentiometry [3]. Thermodynamics and
kinetics [13] were also utilized to study the complexes. Spectrophotometric
techniques applied included FT-IR [16], H-NMR [3, 16], C-NMR [16], and UV-Vis
[3, 16]. From the results of these techniques, information such as the binding
molar ratios [14-16], protonation constants [10, 13, 14], stability constants
[3, 14], and formation constants [5, 10, 11, 13] were determined.
In this study, ultraviolet-visible
spectrophotometry is used to determine the stoichiometry of complex formation
between tyrosine and the transition metal ions Fe(II) and Cu(II). There are
four types of transitions between quantized energy levels that are responsible
for the UV-Vis spectra: σ→σ*, n→σ*, π→π*, and n→π*. The two most important
transitions are π→π* and n→π* because they involve functional groups that are
characteristic of the analyte. Tyrosine�s aromatic group contains mobile π
electrons which correspond to the π→π* transition. This transition has a
characteristic wavelength range of 200-500nm, where the wavelength 274nm is
specific to tyrosine. Several previous studies have utilized UV-Vis
spectrophotometry to measure tyrosine complexation with Al(III), Cu(II), and
Eu(III) ions [3, 10,16]. Since no new peaks were formed by the complex, the
reaction was observed by the enhancement of tyrosine�s absorption at 274nm.
This indicates that the complex absorbs at the same wavelength as tyrosine.
Analysis of the enhancement of
the absorption was carried out via the mole ratio method. One reactant is kept
constant, while the moles of the other reactant are varied. The absorbance is
measured at a wavelength at which the metal-ligand complex absorbs and is then
plotted against the ligand-to metal mole ratio. The mole ratio corresponding to
the intersection of the linear segments indicates the formula of the complex.
This method is also useful for reactions that occur in a stepwise fashion [6].
The mole ratio method was used by Xu and Chen [16] and was also used in this
study in which tyrosine was held constant while varying the moles of metal ion.
The absorbance of the complex was measured at 274nm.
Experimental
Reagents and solutions:
Stock solutions of 1mM FeCl2
(Fisher), CdCl2 (Aldrich), ZnCl2 (Natural Science), and
CuCl2 (Alfa Aesar), and 0.5mM tyrosine (Aldrich) were prepared by
directly dissolving the required amount of substance in distilled water. All
solutions were stored in a refrigerator.
Apparatus:
All spectra were obtained on a SP-2000UV UV-Vis
spectrophotometer. Only the ultraviolet wavelength range (200-400nm) was
utilized.
Procedure:
The absorbance spectrum of tyrosine was scanned
from 200nm to 300nm to ensure that the peak absorbance was found at 274nm, which
is specific for tyrosine. The absorbance spectra of the stock solutions of
transition metal salts were scanned from 260nm to 280nm to check that they did
not absorb at 274nm. Twenty-one sample solutions were made by adding from 0mL
to 2.0mL of 1mM metal salt solution in 0.1mL increments to 2.0mL of 0.5mM
tyrosine and diluting to a total of 4.0mL. All solutions were diluted with
distilled water. The solution�s absorbance was then measured at 274nm with the
UV spectrophotometer. All experiments were carried out at room temperature.
Data and Results
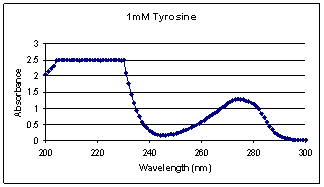
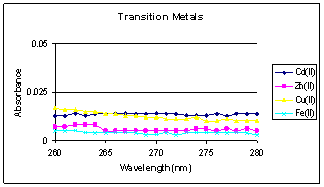 Figure
1 shows the absorption spectrum of 1mM tyrosine; the peak specific to tyrosine
is located at 274nm which is consistent with its literature value. The
absorption spectra of FeCl2, CdCl2, ZnCl2, and
CuCl2 stock solutions are given in figure 2. The stock solutions
showed no significant absorbance.
Figure
1 shows the absorption spectrum of 1mM tyrosine; the peak specific to tyrosine
is located at 274nm which is consistent with its literature value. The
absorption spectra of FeCl2, CdCl2, ZnCl2, and
CuCl2 stock solutions are given in figure 2. The stock solutions
showed no significant absorbance.



A summary of the spectroscopic experimental
data is given in Table 1.
|
|
|
|
|
|
|
Absorbance at 274nm |
|
Sample no. |
Mole Ratio
Tyr:M2+ |
mL
Tyrosine |
[Tyr] x10-4M |
mL M2+ |
[M2+] x10-5M |
Fe(II) |
Cd(II) |
Zn(II) |
Cu(II) |
|
0 |
1:0.0 |
2 |
2.5 |
0.0 |
0.0 |
0.369 |
0.362 |
0.358 |
0.417 |
|
1 |
1:0.1 |
2 |
2.5 |
0.1 |
2.5 |
0.370 |
0.362 |
0.355 |
0.432 |
|
2 |
1:0.2 |
2 |
2.5 |
0.2 |
5.0 |
0.371 |
0.368 |
0.354 |
0.438 |
|
3 |
1:0.3 |
2 |
2.5 |
0.3 |
7.5 |
0.377 |
0.361 |
0.361 |
0.447 |
|
4 |
1:0.4 |
2 |
2.5 |
0.4 |
10.0 |
0.376 |
0.362 |
0.356 |
0.451 |
|
5 |
1:0.5 |
2 |
2.5 |
0.5 |
12.5 |
0.381 |
0.363 |
0.356 |
0.46 |
|
6 |
1:0.6 |
2 |
2.5 |
0.6 |
15.0 |
0.400 |
0.363 |
0.36 |
0.458 |
|
7 |
1:0.7 |
2 |
2.5 |
0.7 |
17.5 |
0.395 |
0.364 |
0.354 |
0.461 |
|
8 |
1:0.8 |
2 |
2.5 |
0.8 |
20.0 |
0.387 |
0.362 |
0.355 |
0.458 |
|
9 |
1:0.9 |
2 |
2.5 |
0.9 |
22.5 |
0.397 |
0.362 |
0.357 |
0.458 |
|
10 |
1:1.0 |
2 |
2.5 |
1.0 |
25.0 |
0.396 |
0.361 |
0.354 |
0.458 |
|
11 |
1:1.1 |
2 |
2.5 |
1.1 |
27.5 |
0.399 |
0.367 |
0.353 |
0.473 |
|
12 |
1:1.2 |
2 |
2.5 |
1.2 |
30.0 |
0.406 |
0.365 |
0.355 |
0.459 |
|
13 |
1:1.3 |
2 |
2.5 |
1.3 |
32.5 |
0.407 |
0.363 |
0.356 |
0.462 |
|
14 |
1:1.4 |
2 |
2.5 |
1.4 |
35.0 |
0.398 |
0.362 |
0.357 |
0.465 |
|
15 |
1:1.5 |
2 |
2.5 |
1.5 |
37.5 |
0.421 |
0.360 |
0.357 |
0.47 |
|
16 |
1:1.6 |
2 |
2.5 |
1.6 |
40.0 |
0.425 |
0.360 |
0.364 |
0.477 |
|
17 |
1:1.7 |
2 |
2.5 |
1.7 |
42.5 |
0.421 |
0.359 |
0.354 |
0.47 |
|
18 |
1:1.8 |
2 |
2.5 |
1.8 |
45.0 |
0.419 |
0.364 |
0.356 |
0.469 |
|
19 |
1:1.9 |
2 |
2.5 |
1.9 |
47.5 |
0.426 |
0.362 |
0.358 |
0.475 |
|
20 |
1:2.0 |
2 |
2.5 |
2.0 |
50.0 |
0.421 |
0.361 |
0.357 |
0.479 |
Upon plotting the spectroscopic data, the following
absorbance vs. mole ratio curves were obtained for the Tyr-Fe(II), Tyr-Cd(II),
Tyr-Zn(II), and Tyr-Cu(II) solutions.
 Figures 3 and 4 show the
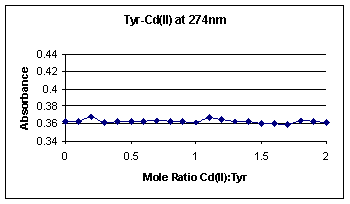
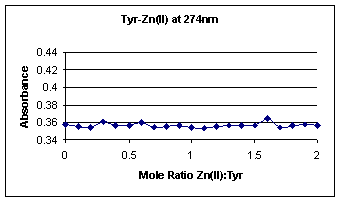
absorbance vs. mole ratio curves for Tyr-Cd(II) and Tyr-Zn(II), respectively.
These two graphs basically show a straight line of constant tyrosine molecule
absorption; the absorption range is only a slight 0.01 difference, as expected.
Therefore, Cd(II) and Zn(II) act as appropriate controls for this experiment.
Figures 3 and 4 show the
absorbance vs. mole ratio curves for Tyr-Cd(II) and Tyr-Zn(II), respectively.
These two graphs basically show a straight line of constant tyrosine molecule
absorption; the absorption range is only a slight 0.01 difference, as expected.
Therefore, Cd(II) and Zn(II) act as appropriate controls for this experiment.

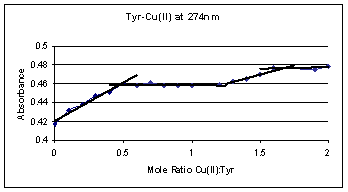

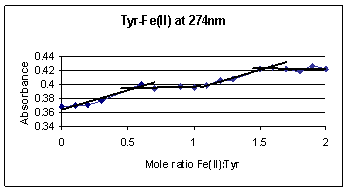
Figure 5 illustrates the complex
formation curve for tyrosine and Fe(II) ions. The absorbance gradually rises
from 0 to 0.5 mole ratio of Fe(II):Tyr, followed by an interval of relatively
constant absorbance. The gradual increase in absorbance up to 0.5 mole ratio
corresponds to a complex formation between two tyrosine molecules and one Fe(II)
ion. 
 Another
gradual increase in absorbance between the mole ratios of 1.0 and1.5 followed by
a plateau is also observed. This similar behavior indicates another complex
formation with two tyrosine molecules and three Fe(II) ions at higher Fe(II)
concentrations. Comparing the absorbance increases of the two mole ratios shows
that the 2:1 Tyr:Fe(II) and 2:3 Tyr:Fe(II) complexes, correspond to a 0.04 and
0.02 increase in absorbance, respectively. The higher increase in absorption
for 2:1 complex implies a greater amount of the 2:1 complex formation.
Another
gradual increase in absorbance between the mole ratios of 1.0 and1.5 followed by
a plateau is also observed. This similar behavior indicates another complex
formation with two tyrosine molecules and three Fe(II) ions at higher Fe(II)
concentrations. Comparing the absorbance increases of the two mole ratios shows
that the 2:1 Tyr:Fe(II) and 2:3 Tyr:Fe(II) complexes, correspond to a 0.04 and
0.02 increase in absorbance, respectively. The higher increase in absorption
for 2:1 complex implies a greater amount of the 2:1 complex formation.
The complex formation curve for
tyrosine and Cu(II) ions is given in figure 6 and is similar to the tyrosine and
Fe(II) formation complex curve in figure 5. The absorbance in figure 6 rapidly
increases from 0 to 0.5 mole ratio and then plateaus. This increase and
leveling off corresponds to two tyrosine molecules coordinating with one Cu(II)
ion. Another increase and leveling occurs at a mole ratio of 1.5 Cu(II):Tyr.
At this mole ratio, a complex of two tyrosine molecules coordinates with three
Cu(II) ions at higher Cu(II) concentrations. The absorbance increases by 0.04
for the 2:1 Tyr:Cu(II) complex, but only increases by 0.02 upon the formation of
the 2:3 Tyr:Cu(II) complex which implies a smaller amount of the 2:3 complex
formation again.
Conclusion and Discussion
This study has shown that the complex of
tyrosine with Fe(II) and Cu(II) ions can be observed using UV spectrophotometry.
Tyrosine exhibits an absorption spectrum in the UV region because it contains
mobile π electrons in its aromatic ring. Excitation occurs when light energy is
absorbed by the electrons in the π bonding orbital causing them to move up to
the π antibonding orbital. A similar effect is taking place in the Cu(II) and
Fe(II)-tyrosine complexes. According to the ligand field theory, coordination
of a ligand like tyrosine disrupts the five-fold degeneracy of transition
metal�s valence d-orbitals. When a field of negative charges from a ligand
surrounds a metal ion, the symmetry of the field is not spherical and therefore
the d-orbital energies split [7]. If the metal ions in figures 7 and 9 are
placed in cubes, the four ligands approaching the metal ion from alternate
corners form a tetrahedral geometry about it. The d-orbitals in a tetrahedral
symmetry split into two closely spaced degenerate energy levels. When the
metal-tyrosine complex absorbs UV light, the electrons in the lower energy d-orbitals
become excited and occupy the higher energy d-orbitals. In addition to the
absorption from the tyrosine molecules, the coordinated metal complex may absorb
light at the same wavelength enhancing the detected absorption. This is the
case observed with Cu(II) and Fe(II) complexes in this study. Cd(II) and Zn(II)
do not display an absorption spectrum due to their completely filled d-orbitals.
The absence of an absorption spectrum makes them suitable control metal ions for
this study.


 Neutral
solutions of pH around 7 are desirable for biological systems because they are
neither too acidic nor too basic. The solutions in this study were prepared at
pH 7, mimicking a biological pH, where the carboxylic acid and amine groups were
significantly deprotonated and the phenolic group was only slightly deprotonated.
This deprotonation allowed for the coordination of the metal ions at these
sites. In tyrosine, as well as other amino acids, the amine and carboxylic acid
functionalities take part in the metal coordination forming a stable five-membered
ring with the metal ion [9]. Cu(II) ions were previously found to bind with the
tyrosine molecule in a 2:1 Tyr:Cu(II) ratio using voltammetry [10, 15]. Results
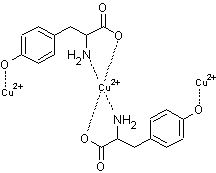
using UV absorption are in agreement with this ratio. The proposed structure of
the 2:1 tyrosine-Cu(II) complex is given in figure 7. The 2:1 Tyr:Cu(II)
complex implies a tetrahedral geometry which is common for transition metal
complexes. However, in this study, evidence for another complex at mole ratio
2:3 Tyr:Cu(II) was also suggested. The proposed structure of the 2:3 tyrosine-Cu(II)
complex is given in figure 8. As seen in figures 7 and 8, two stable five
membered rings are formed between the metal ion, two amino, and two carboxylic
groups. A much smaller amount of complex at 1.5 mole ratio (2:3 Tyr:Cu(II)) is
formed in solution compared to the amount of complex at 0.5 mole ratio (2:1
Tyr:Cu(II)). The small amount of 2:3 Tyr:Cu(II) complex is due to the very
small fraction of deprotonated phenolic group due to a high pKa of
10.5. This study suggests that at high metal concentrations, the possibility of
2:3 Tyr:Cu(II) complex formation.
Neutral
solutions of pH around 7 are desirable for biological systems because they are
neither too acidic nor too basic. The solutions in this study were prepared at
pH 7, mimicking a biological pH, where the carboxylic acid and amine groups were
significantly deprotonated and the phenolic group was only slightly deprotonated.
This deprotonation allowed for the coordination of the metal ions at these
sites. In tyrosine, as well as other amino acids, the amine and carboxylic acid
functionalities take part in the metal coordination forming a stable five-membered
ring with the metal ion [9]. Cu(II) ions were previously found to bind with the
tyrosine molecule in a 2:1 Tyr:Cu(II) ratio using voltammetry [10, 15]. Results
using UV absorption are in agreement with this ratio. The proposed structure of
the 2:1 tyrosine-Cu(II) complex is given in figure 7. The 2:1 Tyr:Cu(II)
complex implies a tetrahedral geometry which is common for transition metal
complexes. However, in this study, evidence for another complex at mole ratio
2:3 Tyr:Cu(II) was also suggested. The proposed structure of the 2:3 tyrosine-Cu(II)
complex is given in figure 8. As seen in figures 7 and 8, two stable five
membered rings are formed between the metal ion, two amino, and two carboxylic
groups. A much smaller amount of complex at 1.5 mole ratio (2:3 Tyr:Cu(II)) is
formed in solution compared to the amount of complex at 0.5 mole ratio (2:1
Tyr:Cu(II)). The small amount of 2:3 Tyr:Cu(II) complex is due to the very
small fraction of deprotonated phenolic group due to a high pKa of
10.5. This study suggests that at high metal concentrations, the possibility of
2:3 Tyr:Cu(II) complex formation.
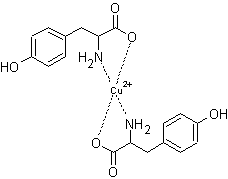
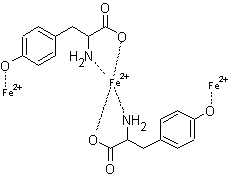
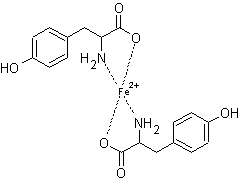
Tyrosine molecules were
previously found to be ligands for Fe(III) ions [4], however the binding of
Fe(II) ions with tyrosine molecules have not been reported. In this study, two
complex formations at the same mole ratios of Cu(II) ions were found for Fe(II)
ions. Fe(II) forms a 2:1 Tyr:Fe(II) complex with tetrahedral geometry. The
structure for the 2:1 complex is shown in figure 9. A complex formation of 2:3
Tyr:Fe(II) was also found. The proposed structure is given in figure 10. Again
a stable five membered ring is formed in both complexes. Similar to Cu(II), the
complex formed at 0.5 mole ratio is much greater than the complex formed at 1.5
mole ratio due to the small percentage of deprotonated phenolic group.


The results of this basic study
may imply some significance in the metabolism and transport mechanisms in
biological systems. The finding of the binding of tyrosine with Fe(II) ions in
the 2:1 Tyr:Fe(II) ratio might help to elucidate the structures of Fe2+
containing active sites in enzymes. Also, the results of this study showed that
both Cu(II) and Fe(II) ions coordinated with tyrosine at a 2:3 Tyr:M(II) ratio,
which has not been reported for any metal ions studied before. Tyrosine might
take part in the metabolism of excess iron and copper in the blood stream by
coordinating with free metal ions, as well as providing a transport system for
copper and iron ions. The results of this study suggest the formation of
interesting new complexes that may prove to be of biological importance.
References:
[1] Berg, Jeremy M.; Tymoczko, John L.; Stryer,
Lubert. Protein Turnover and Amino Acid Catabolism. In Biochemistry, 5;
Susan Moran, Sonia Divittorio, Mark Santee, Georgia Lee Hadler, and Patricia
Zimmermann, Eds.; W. H. Freeman and Company: New York, 2001; 654-655.
[2] Carta, Renzo. Solubilities of L-cystine,
L-tyrosine, L-leucine, and glycine in sodium chloride solutions at various pH
values. J. Chem. Thermodyn 1998, 30, 379-387.
[3] Djurdjevic, Predrag; Jelic, Ratomir; Dzajevic,
Drangana; Cvijovic, Mirjana. Solution Equilibria Between Aluminum (III) Ions and
L-Histidine or L-Tyrosine. Metal Based Drugs 2002, 8,
235-248.
[4] Durmus, Atila; Eichen, Christoph; Sift, Bernd
Horst; Kratel, Andreas; Kappl, Reinhard; Huttermann, Jurgen; Krebs, Bernt. The
active site of purple acid phosphatase from sweet potatoes (Ipomoea batatas):
Metal content and spectroscopic characterization. Eur. J. Biochem.
1999, 260, 709-716.
[5] Ebel, martin; Rehder, Dieter. Vanadium
complexes with enamines having tyrosine constituents. Inorg. Chim. Acta
2003, 356, 210-214.
[6]
Harvey, David. In Spectroscopic Methods of Analysis In Modern
Analytical Chemistry, 1; Kent Peterson and Shirley Oberbroeckling,
Eds.; McGraw Hill Companies, Inc.: Boston, 2002; 406.
[7]
Huheey, James E.; Keiter, Ellen A.; Keiter, Richard L. In Inorganic
Chemistry: Principles of Structure and Reactivity, 4; Jane Piro Ed.;
HarperCollins College Publishers: New York, 1993; pp. 394-403 and 624-630.
[8]
Khan, Farid; Dodke, Ratna. Stability and some Structural Aspects in
Complex Formation between Zinc (II) and Amino Acids and Propionic Adic: A Polarographic
Study. J. Indian Chem. Soc. 1995, 72, 193-198.
[9]
Krishna, Thatavarthy Rama; Jayaraman, Narayanaswamy. Dendritic encapsulation of
amino acid-metal complexes. Synthesis and studies of dendron-functionalized
L-tyrosine-metal (ZnII, CoII) complexes. J.
Chem. Soc., Perkin Trans. 2002, 1, 746-754.
[10]
Letter, John Edward Jr. A Thermodynamic Study of Some Copper(II) and Nickel(II)
Complexes of Amino Acids Related to Serine and Tyrosine. Ph.D. dissertation,
University of Missouri�Columbia, Columbia, MO, Aug. 1969.
[11]
Majid, Sana�; El Rhazi, Mama; Amine, Aziz; Brett, Christopher M. A. An amperometric
method for the determination of trace mercury (II) by formation of complexes
with L-tyrosine. Anal. Chim. Acta 2002, 464, 123-133.
[12]
Richter, Christoph; Azzi, Angelo; Weser, Ulrich; Wendel, Albrecht. Hepatic
Microsomal Dealkylations. J. Bio. Chem. 1977, 252,
5061-5066.
[13]
Ryzhov, Victor; Dunbar, Robert C.; Cerda, Blas; Wesdemoitis, Chrys. Cation-π Effects
in the Complexation of Na+ and K+ with Phe, Tyr, and Trp
in the Gas Phase. Am. Soc. Mass Spectrom. 2000, 11,
1037-1046.
[14]
Sandhu, Ranjit Singh. A Thermodynamic Study of Complexation Reaction
of Yttrium (III), Lanthanum (III) and Cerium (III) with Tyrosine. Montash.
Chem. 1977, 108, 51-55.
[15]
Wang, Lizeng; Ma, Chengsong; Zhang, Xiaoli; Ren, Yiging; Yu, Yong. Determination
of tyrosine traces by adsorption voltammetry of its copper (II) complex. J.
Anal. Chem. 1995, 351, 689-691.
[16] Xu,
Hao; Chen, Liang. Study on the complex site of L-tyrosine with
rare-earth element Eu3+. Spectrochim. Acta, Part A 2003,
59, 657-662.
©
 Figure
1 shows the absorption spectrum of 1mM tyrosine; the peak specific to tyrosine
is located at 274nm which is consistent with its literature value. The
absorption spectra of FeCl2, CdCl2, ZnCl2, and
CuCl2 stock solutions are given in figure 2. The stock solutions
showed no significant absorbance.
Figure
1 shows the absorption spectrum of 1mM tyrosine; the peak specific to tyrosine
is located at 274nm which is consistent with its literature value. The
absorption spectra of FeCl2, CdCl2, ZnCl2, and
CuCl2 stock solutions are given in figure 2. The stock solutions
showed no significant absorbance.







